The COVID-19 vaccine trial marks a landmark initiative in the race against the pandemic, enabled by the UK Government’s approval. Set to begin in January 2021, this revolutionary “human challenge trial” involves meticulously infecting paid volunteers with the Coronavirus to expedite vaccine efficacy research. Supported by experts from institutions like Imperial College London, this trial represents a bold step in the ongoing COVID-19 vaccine program, with £33.6 million allocated to ensure its success. The first phase will identify the minimum viral dose required for infection, setting the foundation for subsequent phases of vaccination. With results anticipated by May 2021, this trial could significantly influence global vaccination strategies and responses to COVID-19.
In the context of the pandemic, the recently authorized vaccine investigation seeks to optimize vaccine development through controlled exposure of selected participants. As a pivotal part of the strategy to combat the Coronavirus, this innovative research initiative—often referred to as a challenge study—aims to assess vaccine safety and effectiveness under monitored conditions. The initiative involves a cohort of young, compensated volunteers who will undergo rigorous testing procedures designed by researchers at leading institutions, including challenges noted by the UK Government regarding vaccine deployment. Engaging such methodologies underscores a significant evolution in public health efforts that prioritize expedited solutions while addressing ethical considerations inherent in medical trials. Ultimately, this trial not only emphasizes the urgency of the global health crisis but also highlights the commitment to scientific advancement in the face of adversity.
Understanding COVID-19 Vaccine Trials
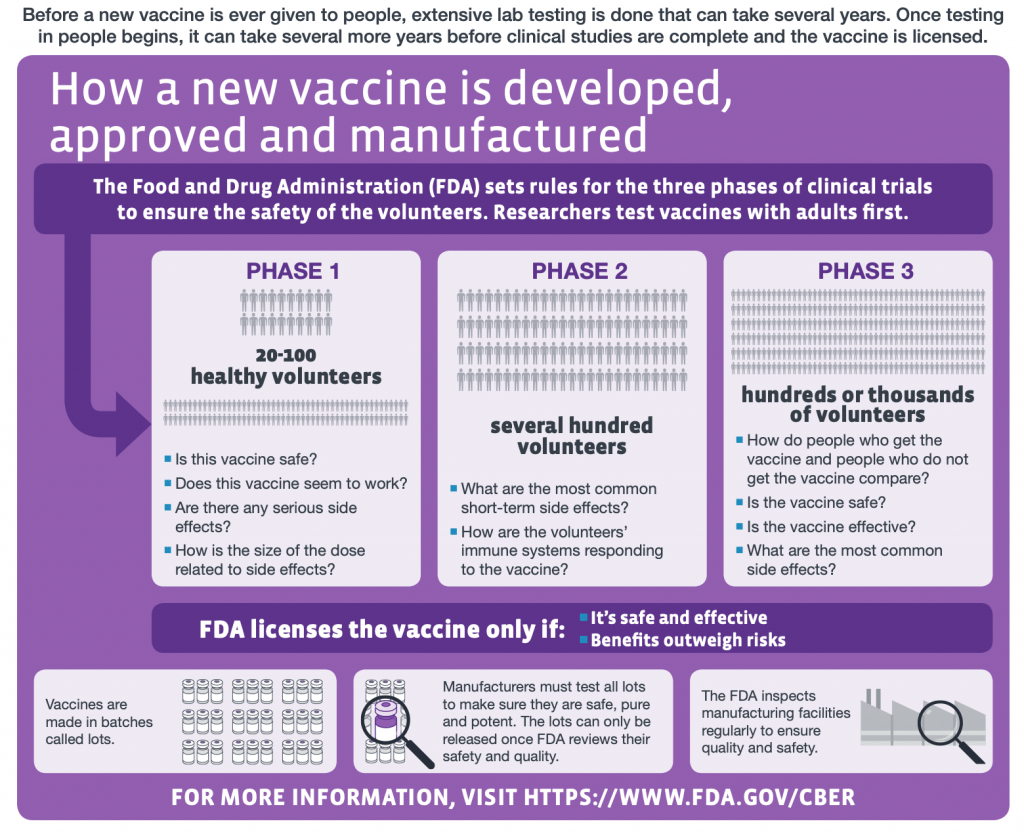
COVID-19 vaccine trials are an essential part of the global effort to mitigate the pandemic’s impact. They are meticulously designed studies that test the safety and efficacy of vaccines in human subjects. In the UK, the government has recently approved a novel approach known as a “human challenge trial,” where healthy volunteers are intentionally infected with the Coronavirus to test the vaccine’s effectiveness. This innovative method aims to speed up the vaccine development process, thereby addressing the urgent need for solutions to combat COVID-19.
The importance of such trials lies in their ability to provide rapid and reliable data on vaccine performance. By understanding how different vaccines stimulate the immune response when exposed to the virus, researchers can fine-tune formulations and dosing protocols. This aspect is especially vital for the COVID-19 vaccine program, as the world looks forward to achieving herd immunity and safely returning to normalcy.
The Role of the UK Government in Vaccine Approvals
The UK Government plays a crucial role in the oversight and funding of vaccine trials. By allocating significant financial resources, such as the £33.6 million designated for the COVID-19 vaccine program, it aims to bolster research and development efforts. This commitment has enabled various institutions, including Imperial College London, to conduct trials that are crucial for public health.
Furthermore, the government’s approval processes ensure that vaccine trials maintain ethical standards and safety protocols, especially when they involve potentially risky procedures like human challenge trials. Such diligence reassures the public about the reliability of the vaccines being developed, helping to build trust in the vaccination process.
The Human Challenge Trial Explained
The human challenge trial model represents a significant shift in how vaccine efficacy can be tested. In these trials, paid volunteers aged between 18 and 30 are selected to take part in a carefully monitored study. The first phase involves administering a specific dose of the Coronavirus to determine the minimal infectious dose required to elicit disease in a controlled environment. This phase will provide foundational data that will guide subsequent steps in the vaccine development process.
The goal of the human challenge trial is to accelerate the pace at which effective vaccines can be developed. By having volunteers exposed to the virus post-vaccination, researchers can quickly assess how well the vaccines confer immunity and protection against COVID-19, thus facilitating the urgent need for solutions amidst the ongoing pandemic.
Involvement of Paid Volunteers in COVID-19 Trials
Paid volunteers are critical to the success of COVID-19 vaccine trials, including the human challenge trials. These individuals are usually compensated for their time, effort, and the risks they undertake by participating in research that could lead to significant public health benefits. The recruitment process is highly regulated, ensuring that volunteers are well-informed about potential risks and benefits.
In the UK, the response from potential volunteers has been encouraging, with many willing to contribute to the advancement of science and the fight against the pandemic. Their involvement is essential not only from an ethical standpoint but also because it allows researchers to gather diverse data from different demographic groups, ultimately leading to more effective vaccines.
The Impact of the Imperial College London Trial
The collaboration between the UK Government and leading institutions like Imperial College London underscores the significance of scientific research in combating COVID-19. This trial is particularly noteworthy due to its innovative approach and the potential insights it can offer into the dynamics of viral infection and immunity. The trial aims to explore various aspects of the vaccine’s effectiveness in real-time and under controlled conditions.
Researchers at Imperial College London believe that their findings will not only contribute to the development of successful COVID-19 vaccines but also enhance understanding of viral transmission. The data gathered from this trial could have far-reaching implications for pandemic preparedness and future vaccine strategies.
Ethical Considerations in COVID-19 Challenge Trials
Human challenge trials, while being innovative, raise ethical questions about the risks posed to participants. Critics argue that intentionally infecting healthy volunteers may expose them to unnecessary danger. However, proponents maintain that these trials are well-managed and that the potential benefits to society far outweigh the risks involved. Ethical oversight from the relevant committees is critical in ensuring that participants are well-informed and consenting.
Moreover, discussions surrounding the ethical implications of human challenge trials have become increasingly relevant as scientists strive to balance urgent public health needs with individual participant safety. Ensuring rigorous ethical standards in these trials is vital for maintaining public trust and encouraging participation.
Comparative Analysis of Traditional Trials vs. Challenge Trials
Traditional vaccine trials typically involve large, diverse populations being monitored over extended periods to assess the safety and efficacy of the vaccine post-administration. In contrast, human challenge trials expedite this process by allowing researchers to deliberately expose vaccinated volunteers to the virus. This streamlined approach potentially offers quicker insights into vaccine performance and can significantly reduce the timeline for approval.
However, while challenge trials can facilitate faster results, they are not without controversy. The benefits versus risks must be carefully weighed, especially in the case of a new virus like COVID-19. The ongoing dialogue in the scientific community about the merits of both traditional and challenge trials contributes to shaping future guidelines for vaccine research and public health practice.
Funding and Support for the UK COVID-19 Vaccine Program
The UK Government’s commitment to providing financial support for the COVID-19 vaccine program reflects its priority in addressing the pandemic’s consequences. The funding not only allows for the human challenge trial to proceed, but it also facilitates broader research initiatives aimed at developing and distributing vaccines more rapidly. The investment underscores the public health strategy to safeguard lives through scientific innovation.
In addition to government support, collaboration with private organizations and research institutes has intensified efforts in vaccine development. Such partnerships can bring additional expertise and resources, bolstering the efficacy of research programs. The continuation of this funding strategy is crucial for maintaining progress in the UK’s battle against COVID-19.
Public Response to the COVID-19 Vaccine Program
Public perception regarding the COVID-19 vaccine program has varied significantly. While there is considerable optimism about the development of vaccines, some individuals express concerns about the safety and ethical implications of trials, particularly human challenge trials. Effective communication from health authorities and researchers is essential in assuaging fears and disseminating accurate information about vaccine safety.
Engagement strategies aimed at fostering public trust, such as transparency in trial processes and outcomes, are necessary for encouraging vaccine uptake. Overcoming hesitance and misinformation can significantly impact the overall efficacy of vaccination efforts, which are vital for achieving community immunity against COVID-19.
Frequently Asked Questions
What is a COVID-19 vaccine trial and how does it differ from regular vaccine trials?
A COVID-19 vaccine trial is a research study designed to evaluate the safety and efficacy of new vaccines against the Coronavirus. Unlike standard trials, the UK’s human challenge trial intentionally infects paid volunteers with the virus to observe the vaccine’s effects under controlled conditions. This approach aims to accelerate the COVID-19 vaccine program by providing quick insights into vaccine effectiveness.
How does the UK’s human challenge trial for the COVID-19 vaccine work?
The UK Government-approved human challenge trial for the COVID-19 vaccine will start by infecting paid volunteers aged 18 to 30 with a controlled dose of Coronavirus. Conducted by experts from Imperial College London, the trial will first identify the minimum infectious dose and then assess vaccine responses by exposing vaccinated individuals to the virus. The aim is to evaluate vaccine performance more rapidly than traditional methods.
What are the ethical considerations surrounding the COVID-19 vaccine trial involving paid volunteers?
The COVID-19 vaccine trial raises important ethical questions, especially since it involves deliberately infecting paid volunteers. Critics argue that this poses unnecessary risks, while supporters believe it is crucial for advancing the COVID-19 vaccine program. The trial’s design is overseen by an ethics committee to ensure participant safety and informed consent, balancing scientific advancement with ethical accountability.
Who is sponsoring the COVID-19 vaccine trial in the UK and how much funding has been allocated?
The UK Government is sponsoring the COVID-19 vaccine trial, allocating £33.6 million ($43.5 million) to support the initiative. This funding is part of a broader COVID-19 vaccine program aimed at developing and testing effective vaccines, facilitated by organizations such as hVIVO and the Royal Free London NHS Foundation Trust.
What is the expected timeline for the results of the COVID-19 vaccine program’s human challenge trial?
The human challenge trial for the COVID-19 vaccine program in the UK is expected to begin in January 2021, pending final ethical approvals. Researchers anticipate results by May 2021, which will inform the next steps in the vaccine development process and assess the trade-off between potential risks and scientific benefits.
What role does Imperial College London play in the COVID-19 vaccine trial?
Imperial College London is a key participant in the COVID-19 vaccine trial, providing expertise and research capabilities for the human challenge trial. Researchers from the institution are working collaboratively to ensure that the trial adheres to safety protocols while expediting the evaluation of vaccine effectiveness against the Coronavirus.
Why have human challenge trials for COVID-19 faced opposition?
Human challenge trials for COVID-19 have faced opposition due to concerns regarding the safety of deliberately infecting volunteers with a virus that is already widespread. Critics argue that the risks may outweigh the potential benefits, while proponents highlight the urgent need for accelerated vaccine development in the face of the pandemic. Balancing these viewpoints is essential for ethical trial execution.
What is the significance of the COVID-19 vaccine trial for future vaccines?
The COVID-19 vaccine trial, particularly the human challenge trial model, is significant as it could pave the way for faster vaccine development against various infectious diseases. The insights gained from this trial may inform strategies for future vaccine programs, improve methods of testing efficacy, and expedite responses to emergent public health challenges.
| Key Point | Details |
|---|---|
| Trial Approval | The UK Government approved the COVID-19 vaccine trial on October 20, 2020. |
| Trial Details | The trial involves intentionally infecting volunteers with the Coronavirus to study vaccine efficacy. |
| Funding | £33.6 million ($43.5 million) allocated for the COVID-19 vaccine program. |
| Volunteer Age Group | Participants will be paid volunteers aged 18 to 30. |
| Trial Phases | Initial phase to identify the minimum dose of Coronavirus; subsequent phases involve vaccination and exposure to the virus. |
| Timeline | Trial set to begin in January 2021 with expected results by May 2021. |
| Expert Oversight | The trial supervised by experts from hVIVO and the Royal Free London NHS Foundation Trust. |
| Opposition and Support | While there is opposition due to ethical concerns, proponents argue challenge trials could accelerate vaccine development. |
| Scientific Objective | Aim to validate trade-offs between potential risks and scientific benefits in developing COVID-19 vaccines. |
Summary
The COVID-19 vaccine trial approved by the UK Government marks a significant advancement in the battle against the pandemic. Initiating in January 2021, this human challenge trial will intentionally expose volunteers to the Coronavirus to better understand vaccine efficacy and responses. With a substantial investment of £33.6 million allocated for the trial, it aims to swiftly facilitate the development of effective treatments and vaccines against COVID-19. Despite some ethical concerns, the collaboration between esteemed institutions reflects an urgent need to accelerate scientific progress in combating the virus. Anticipated results by May 2021 promise to inform future strategies in the ongoing fight against COVID-19.